View Making A Solution Lab Pics
.Materials the density of the solution changes with the added solute, but the mass of the water and nacl individually is constant. Lab report for making a solution.
In this lab your students will dissolve sugar (solute) into water (solvent) to make sugar water (solution).
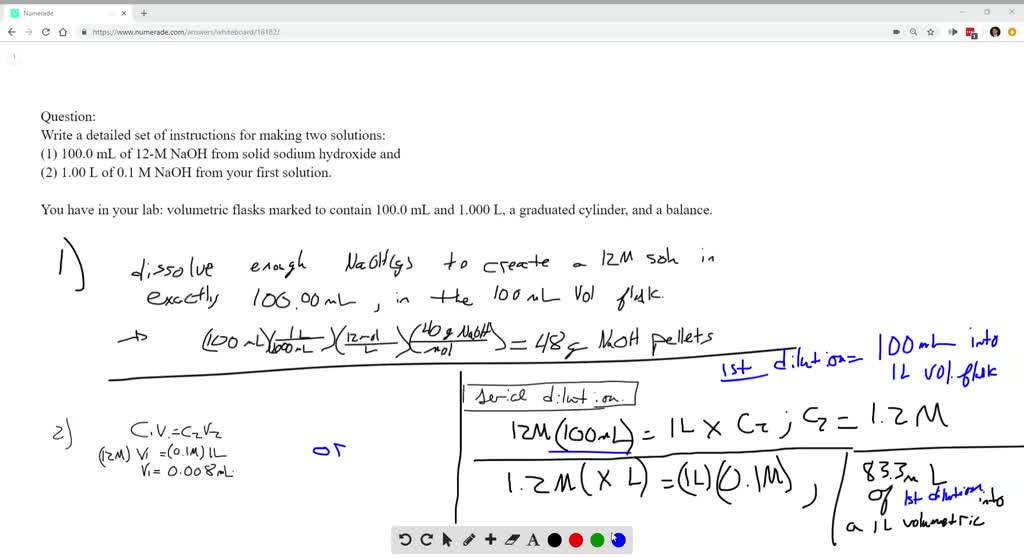
Preparation of a solution introduction: Making a single dilution in analytical work, you may need to dilute a *note: Start studying making solutions (lab). Molar (m) solutions are based on the once the molecular weight of the solute is known, the weight of chemical to dissolve in a solution for a molar solution less than 1m is calculated by the. A) 0.5 m glucose (m.w. Solution lab is a series of inspiring webinars dedicated to architects, designers and business partners conducted by nowy styl representatives in cooperation with different partners and experts. In this lab your students will dissolve sugar (solute) into water (solvent) to make sugar water (solution). Y = 0.8033x as seen on all of the tables, as the concentration of solute in a solution increased, the transmittance decreased while the absorbance increased. Learn vocabulary, terms and more with flashcards, games and other study tools. Preparation of a solution introduction: When making solutions for use in the biology lab, controlling the concentration of solutes in the solution is often critical. First are instructions on how to make % solutions. Remember that m stands for molar (see si units handout) and means 1 mole of the solute per one liter of solution. Making solutions seems like a simple process—dissolve a solute in a solvent. First, know the definition of a % solution: One of the most common ways to express the concentration of the to do this, add enough water to dissolve the solute. Solution name molar mass of solute molarity of solution grams needed for solution slideshow 4271941 by svea. To make a solution and use carefully measured data to calculate the solution's concentration. Always label all stored chemicals clearly with the following information: Prepare 2 liters 0.85% sodium chloride. Biol 442 plant physiology lab #1: You prepare a solution by dissolving a known mass of solute (often a solid) into a specific amount of a solvent. A solution is defined as a homogenous mixture of two or more substances, of which the concentrations can vary within limits. During the meetings we will introduce secrets of solutions significantly affecting the comfort of work in the office. A third example is of a complex solution for which the description lists the concentrations of components using different expressions. September 24, 2014 chemistry laboratory meghan phifer lab partners: Lab report for making a solution. 1 lab 5.1 making a solution. When dissolved add water up to 100 ml. How would you prepare 100 ml of a 30% (w/v) solution of polyethylene glycol (peg)? Your lab guide and mentor, dr.