37+ Conductivity Of Aqueous Solutions And Conductometric Titrations Lab Pics
.Titrant volume values to a new method of analysis of conductometric titration data (coelho, l.h.g. During many titrations, the conductivity changes significantly.
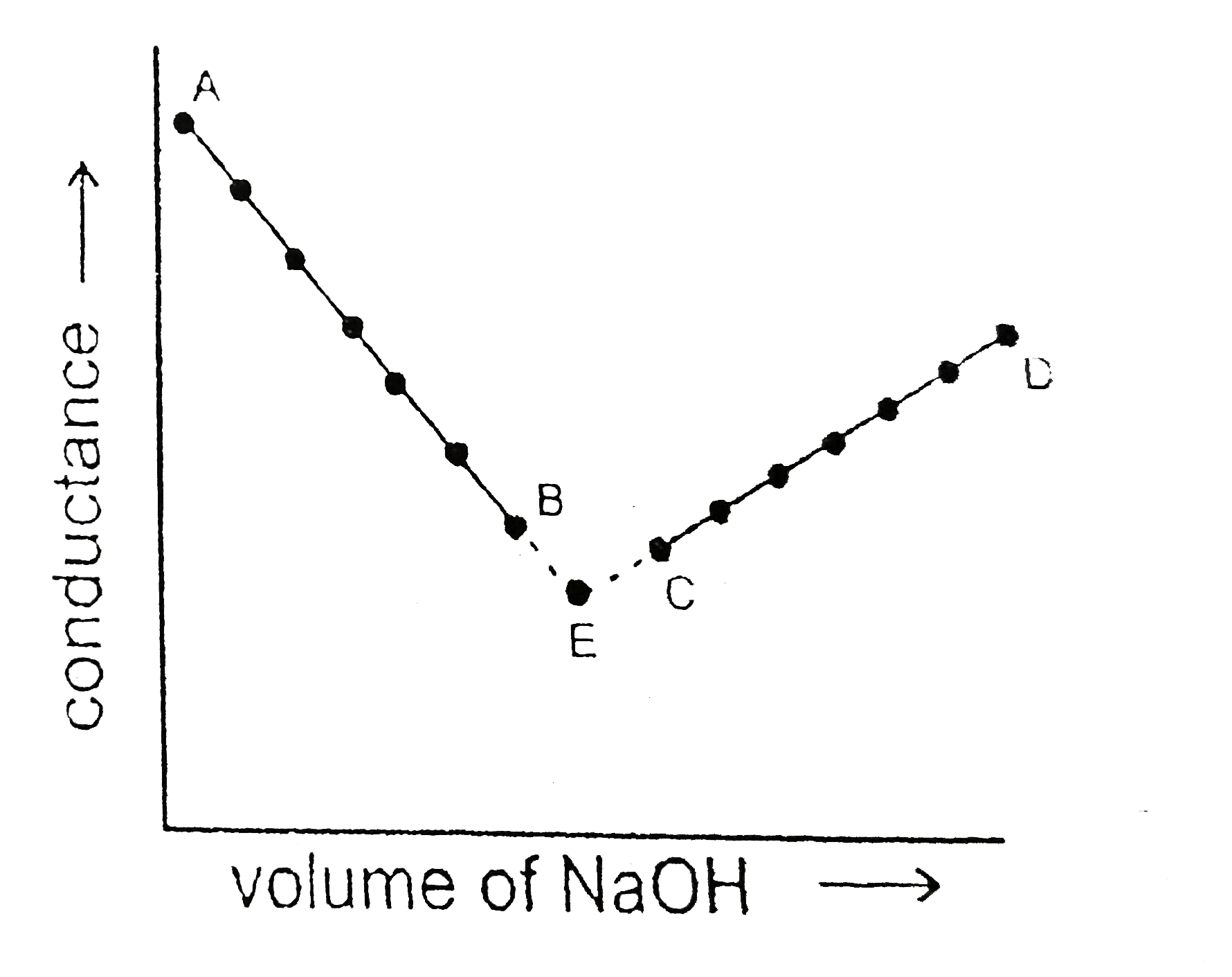
You may have access to conductivity cells in your lab that have been designed for conductometric titrations.
The conductivity of a solution depends on the ions present in it. During many titrations, the conductivity changes significantly. The conductivity of a solution depends on the ions present in it. The principle of conductometric titration. You may have access to conductivity cells in your lab that have been designed for conductometric titrations. During the titration, one of the ions is replaced by the other and invariably these two ions differ in the ionic conductivity with the result that conductivity of the solution varies during the course of titration. If you do not have access to that kind of equipment, then you could use the apparatus shown below to measure the changes in conductance of a solution as base in the burette is added to acid in the beaker (or, as acid in the burette is. Titrant volume values to a new method of analysis of conductometric titration data (coelho, l.h.g. We have used curtipot, e.g., to simulate and feed ph vs.